Separation of Polypeptides
Get In Touch
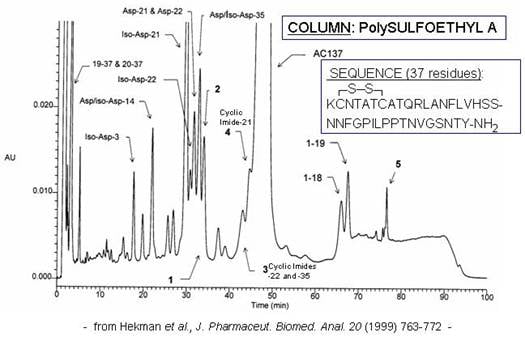
Synthetic polypeptides : These too can experience deamidation and dehydration, in addition to missing residues and nonenzymatic cleavage. The selectivity of ion-exchange HPLC nicely complements that of reversed-phase (RPC), often resolving variants that coelute in RPC. The example below shows the analysis of pramlintide® in an accelerated heat stress test. Pramlintide is a synthetic analog of the pancreatic peptide amylin, which complements the action of insulin in regulating the metabolism of glucose. Pramlintide’s sequence is given below in the figure.
All asparagine residues are potential deamidation sites. In this severely degraded sample, a deamidation product was observed for each such site except for Asn31.
Application Name : Synthetic polypeptides
Column Name : PolySULFOETHYL A™ Columns
Analytes : Polypeptides
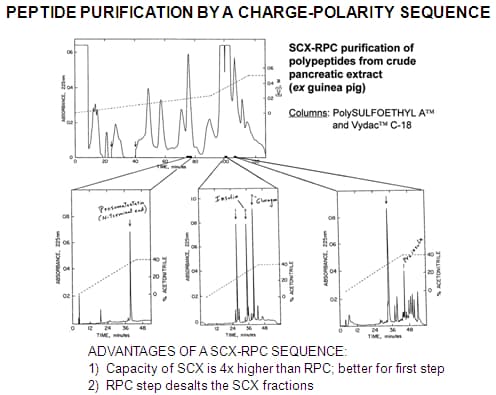
Generally, any single protein or peptide can be isolated in >90% purity from any crude extract by the use of two complementary modes of HPLC in sequence. If purity > 98% is desired, it may be necessary to use three modes in sequence.
PEPTIDES :
SCX-RPC : Almost all peptides will be retained by our PolySULFOETHYL A™ material at pH 2.7-3.0 and can be separated by charge differences. The fraction of interest is collected and further resolved by RPC. Peptides which coeluted with the peptide of interest in the SCX run and which presumably have similar charge-to-mass characteristics will be unlikely to have similar polarity as well and will be separated in the second dimension.
Order PolySULFOETHYL A™ now.
The SCX step should be performed first for the following reasons :
1) Ion-exchange columns have 4x higher binding capacity than a RPC column of the same size.
2) The RPC step can conveniently eliminate the salt from the SCX step.
Application Name : Purification Of Peptide By A Charge Polarity Sequence
Column Name : PolySULFOETHYL A™ Columns
Analytes : Polypeptides
RPC-HILIC-SCX: This sequence was used to obtain polypeptides of extremely high purity from crude pituitary extracts for correction of mistaken sequence data in the literature (actually, a SCX-RPC-HILIC sequence would have been more convenient). Ref: K.F. Faull et al., Neuropeptides 32 (1998) 339.
PROTEINS :
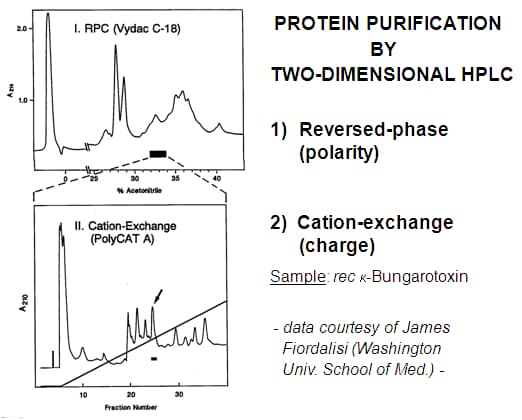
Ion-exchange and hydrophobic interaction chromatography (HIC) are useful for most protein applications, particularly if protein tertiary structure and function is to be preserved (not a concern in proteomics). The capacity and resolving power of SEC is much less than for these modes, making it useful only in special cases. The organic solvents used for RPC and HILIC also make them useful only for special cases. Small proteins tend to work well with RPC while proteins < 30 KDa and membrane proteins frequently work well with HILIC.
RPC-WCX : This combination worked well for this small protein. It would have been more convenient to use a WCX-RPC sequence.
Application Name : Purification Of Protein By Two-Dimensional HPLC
Column Name : PolyCAT A™ Columns
Analytes : Polypeptides
Get Your Quote or Call: 040-29881474
We focus on supporting laboratory workflows & optimizing lab-wide operations
