
High Performance Liquid Chromatography (HPLC) is a powerful analytical technique used widely in pharmaceutical, chemical, environmental, and food industries. Known for its precision and versatility, HPLC plays a vital role in separating, identifying, and quantifying components in complex mixtures. In this blog post, we’ll explore the fundamentals of HPLC, how it works, and why it’s essential in modern analytical laboratories.
What is HPLC?

HPLC, short for High Performance Liquid Chromatography, is an advanced form of column chromatography. It utilizes high pressure to push a liquid sample through a tightly packed column filled with solid adsorbent material. Each compound in the sample interacts differently with the stationary phase, allowing for precise separation based on polarity, size, or charge.
How Does High Performance Liquid Chromatography Work?
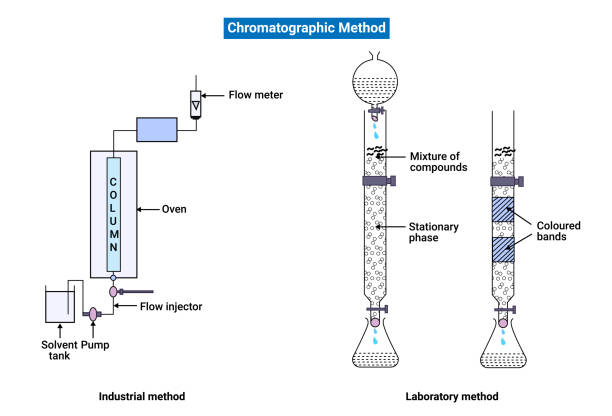
The core principle of High Performance Liquid Chromatography involves the movement of a liquid mobile phase through a column containing a solid stationary phase. As the sample travels through the column:
- Compounds interact with the stationary phase at different rates
- Retention time (the time a compound takes to exit the column) varies
- A detector records and displays the separated components as peaks on a chromatogram.
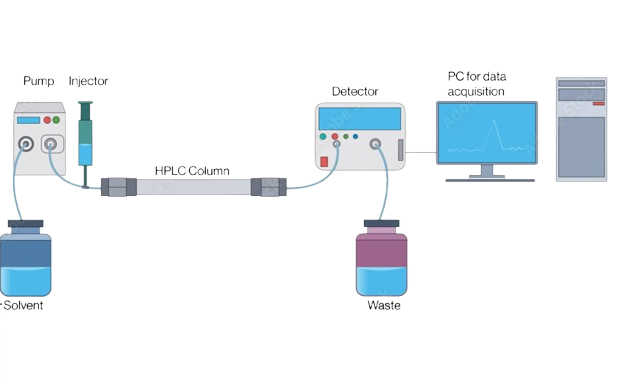
Main Components of an HPLC System:
- Pump: Delivers mobile phase at constant flow
- Injector: Introduces the sample
- Column: Performs the separation
- Detector: Identifies and quantifies analytes
- Data System: Records and analyzes results
Applications of HPLC

HPLC is widely used across industries for both qualitative and quantitative analysis. Common applications include:
- Pharmaceuticals: Drug purity testing, stability studies, impurity profiling
- Environmental Analysis: Water, soil, and air contaminant detection
- Food & Beverage: Additive analysis, sugar profiling, pesticide residues
- Forensic Science: Toxicology, drug identification, and fingerprint analysis
- Biotechnology: Protein, peptide, and nucleotide separation
Types of HPLC Techniques

There are several types of High Performance Liquid Chromatography techniques based on the nature of the stationary and mobile phases:
- Reverse Phase HPLC (RP-HPLC) – Most common, uses a non-polar stationary phase.
- Normal Phase HPLC (NP-HPLC) – Uses a polar stationary phase.
- Ion Exchange HPLC – Separates ionic species.
- Size Exclusion HPLC (SEC) – Based on molecular size.
Each technique is suited for specific types of analytes and separation needs.
Advantages of HPLC

- High accuracy and reproducibility
- Fast and efficient separation
- Applicable to a wide range of compounds
- Quantitative and qualitative capabilities
- Minimal sample preparation
Due to these advantages, High Performance Liquid Chromatography remains a preferred method in regulated industries requiring strict compliance and reliability.
Choosing the Right HPLC Column

The choice of HPLC column significantly affects the quality of separation. Factors to consider include:
- Stationary phase chemistry (e.g., C18, C8, PFP, amino)
- Particle size and pore size
- Column length and inner diameter
- Sample type and mobile phase compatibility
Selecting the right column ensures optimal resolution, retention time, and peak shape.
Final Thoughts
HPLC has revolutionized chemical analysis by offering unmatched precision and efficiency. Whether you’re working in pharma, food testing, environmental science, or academic research, High Performance Liquid Chromatography provides the tools needed to ensure accurate and reliable results.
To get the most out of your HPLC analysis, choose the right instrumentation, columns, and techniques suited to your specific application.
Frequently Asked Questions (FAQ)
Q1. What is the principle of HPLC?
HPLC works on the principle of differential partitioning between a mobile phase and a stationary phase.
Q2. Is HPLC quantitative or qualitative?
HPLC is both. It provides quantitative data (e.g., concentration) and qualitative insights (e.g., identity of compounds).
Q3. What does a chromatogram show?
A chromatogram displays peaks representing separated analytes, each corresponding to different compounds in the sample.
