Get In Touch
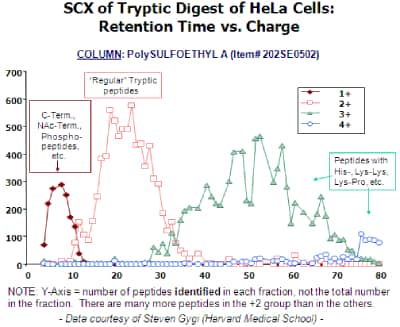
Isolation of Phosphopeptides and Other Classes of Peptides from Tryptic Digests: The typical tryptic peptide has a net charge of +2 at pH 2.7-3.0, due to the N-terminus and the Lys or Arg residue at the C-terminus. Attachment of a phosphate group lowers the net charge of the peptide to +1. Thus, the earliest-eluting SCX fractions are enriched in phosphopeptides, as well as C-terminal and blocked N-terminal fragments. The 200-Å pore version of PolySULFOETHYL A™ has a higher surface area than the 300-Å material normally used for proteomics and can pull the +2 peptides away from the +1 peptides reasonably completely [ABOVE]. Beausoleil et al. (PNAS 101 (2004) 12130) have used this approach to identity over 2000 phosphopeptides from the tryptic digest of HeLa cell lysate. In various studies, about 20-30% of the phosphopeptides in a really complex tryptic digest eluted in this +1 window. A good recent examination of this approach is: J.C. Trinidad et al., Mol. Cell. Proteomics 5 (2006) 914.
Column Name : PolySULFOETHYL A™ Columns
Compound Name : Phosphopeptides
Get Your Quote or Call: 040-29881474
We focus on supporting laboratory workflows & optimizing lab-wide operations
