Separation of EDTA (Ethylenediaminetetraacetic Acid)
Get In Touch
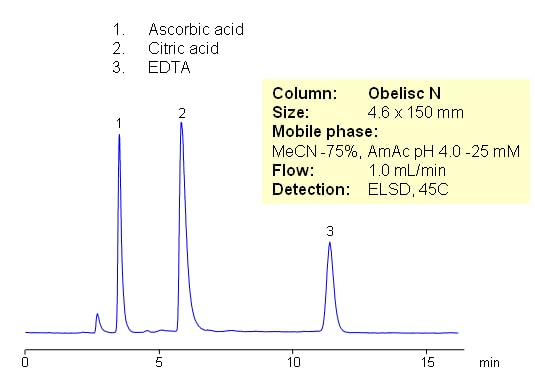
Citric acid, ascorbic acid and EDTA are commonly used in food and pharmaceutical industry as preservatives. These compounds are very polar in nature. They are weak organic acids with limited UV activity. Retention and separation is achieved on HILIC mixed-mode Obelisc N column. All three compounds are retained by combination of strong HILIC and strong anion-exchange mechanisms. Separation can be monitored by ELSD, LC/MS, UV or Corona CAD. In contrast to other HILIC column, Obelisc N has two ionizable groups basic and acidic which provide ion-exchange interaction in addition to hydrophilic interaction. This allows to use less acetonitrile for HILIC separation.
Application Name : HILIC Separation of Common Preservatives – Citric Acid, Ascorbic Acid and EDTA
Column Name : Obelisc N Columns
Analytes : Citric acid, Ascorbic Acid, EDTA (Ethylenediaminetetraacetic Acid)
Application Name : HILIC Separation of Common Preservatives – Citric Acid, Ascorbic Acid and EDTA
Get Your Quote or Call: 040-29881474
We focus on supporting laboratory workflows & optimizing lab-wide operations
