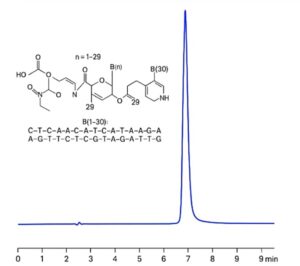
Description
Eteplirsen (Exondys 51) is an antisense oligonucleotide designed for treating certain forms of Duchenne muscular dystrophy (DMD), a rare genetic condition marked by progressive muscle weakness. It is specifically indicated for patients with a confirmed DMD gene mutation amenable to exon 51 skipping.
Mechanism of Action: Eteplirsen binds to exon 51 of dystrophin pre-mRNA, promoting its exclusion during mRNA processing. This results in the production of a shortened but functional dystrophin protein.
Approval: The U.S. FDA granted Eteplirsen accelerated approval in 2016 based on observed increases in dystrophin levels. Ongoing clinical trials are required to verify its long-term clinical benefit.
Administration: Eteplirsen is delivered through intravenous infusion.
Structure: As an oligonucleotide, Eteplirsen is composed of a specific sequence of nucleic acids.
Safety and Efficacy: Common side effects include balance issues and vomiting. Its effect on improving motor function is still under investigation.
Analytical Method:
Eteplirsen can be retained and analyzed using a Zodiac HST B mixed-mode stationary phase column. The method utilizes an gradient mobile phase consisting of water, acetonitrile (MeCN), and sulfuric acid as a buffer, with detection performed by UV at 260 nm.
Condition
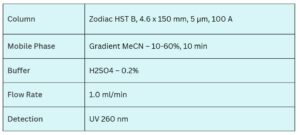
Column Name : Zodiac HST B
Compound Name : Eteplirsen
Get Your Quote or Call: 040-29881474
We focus on supporting laboratory workflows & optimizing lab-wide operations
