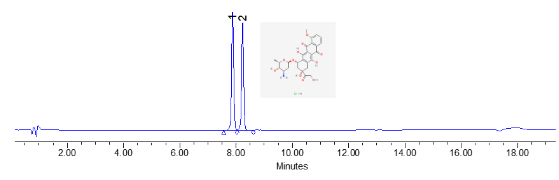
The application of Doxorubicin HCl and Epirubicin HCl using the API_USP method with a C18 column involves their precise analysis and quantification in pharmaceutical formulations. Employing high-performance liquid chromatography (HPLC) with a C18 stationary phase, as specified by the United States Pharmacopeia (USP), ensures accurate separation and detection of these chemotherapeutic agents. This methodological approach guarantees compliance with regulatory standards regarding the identity, strength, quality, and purity of the drugs, essential for their safe and effective use in cancer treatment. By following these established protocols, pharmaceutical scientists and healthcare providers can confidently assess and maintain the potency and integrity of Doxorubicin HCl and Epirubicin HCl formulations, supporting optimal therapeutic outcomes for patients.
Condition
Description
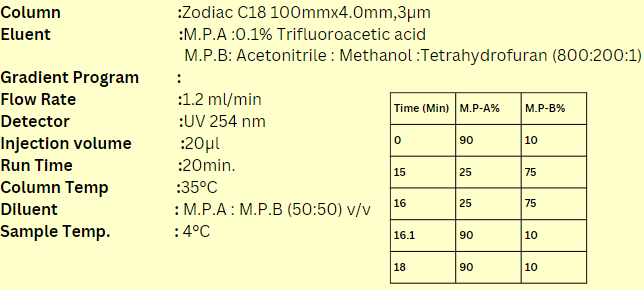
| Column Name | Zodiac C18 Column |
| Compound Name | 1)Doxorubicin Hcl 2)Epirubicin Hcl |
Get Your Quote or Call: 040-29881474
We focus on supporting laboratory workflows & optimizing lab-wide operations
