Description
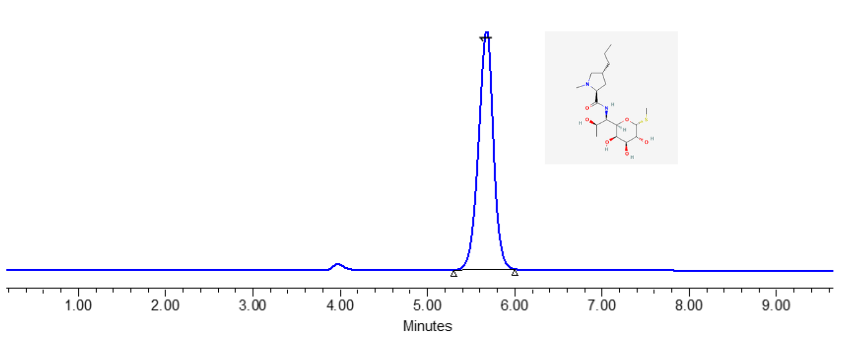
The assay of Lincomycin Injection according to the USP (United States Pharmacopeia) involves a specific methodology to ensure the correct concentration and quality of the drug in the injection. While I can’t provide the exact proprietary details of USP methods. For the specific USP method, including details such as reagent preparation, exact chromatographic conditions, and calculation procedures, you should refer to the latest USP monograph for Lincomycin Injection. This monograph will provide precise and detailed instructions that must be followed to comply with USP standards. If you have access to the USP, you can find the complete method in the Lincomycin Injection section of the USP monograph.
Condition
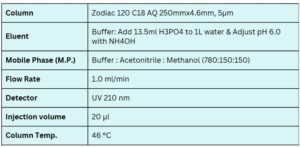
Column Name : Zodiac 120 C18 AQ HPLC Column
Compound Name : Lincomycin
Lincomycin
Get Your Quote or Call: 040-29881474
We focus on supporting laboratory workflows & optimizing lab-wide operations